Immunostaining - Immunofluorescence
- Immunofluorescence
- Fluorescent Microscope
- IF history
- Indirect and direct IF
- IF Examples
Immunofluorescence is a technique used for light microscopy with a fluorescence microscope and is used primarily on microbiological samples. This technique uses the specificity of antibodies to their antigen to target fluorescent dyes to specific biomolecule targets within a cell, and therefore allows visualisation of the distribution of the target molecule through the sample. Immunofluorescence is a widely used example of immunostaining and is a specific example of immunohistochemistry that makes use of fluorophores to visualise the location of the antibodies.
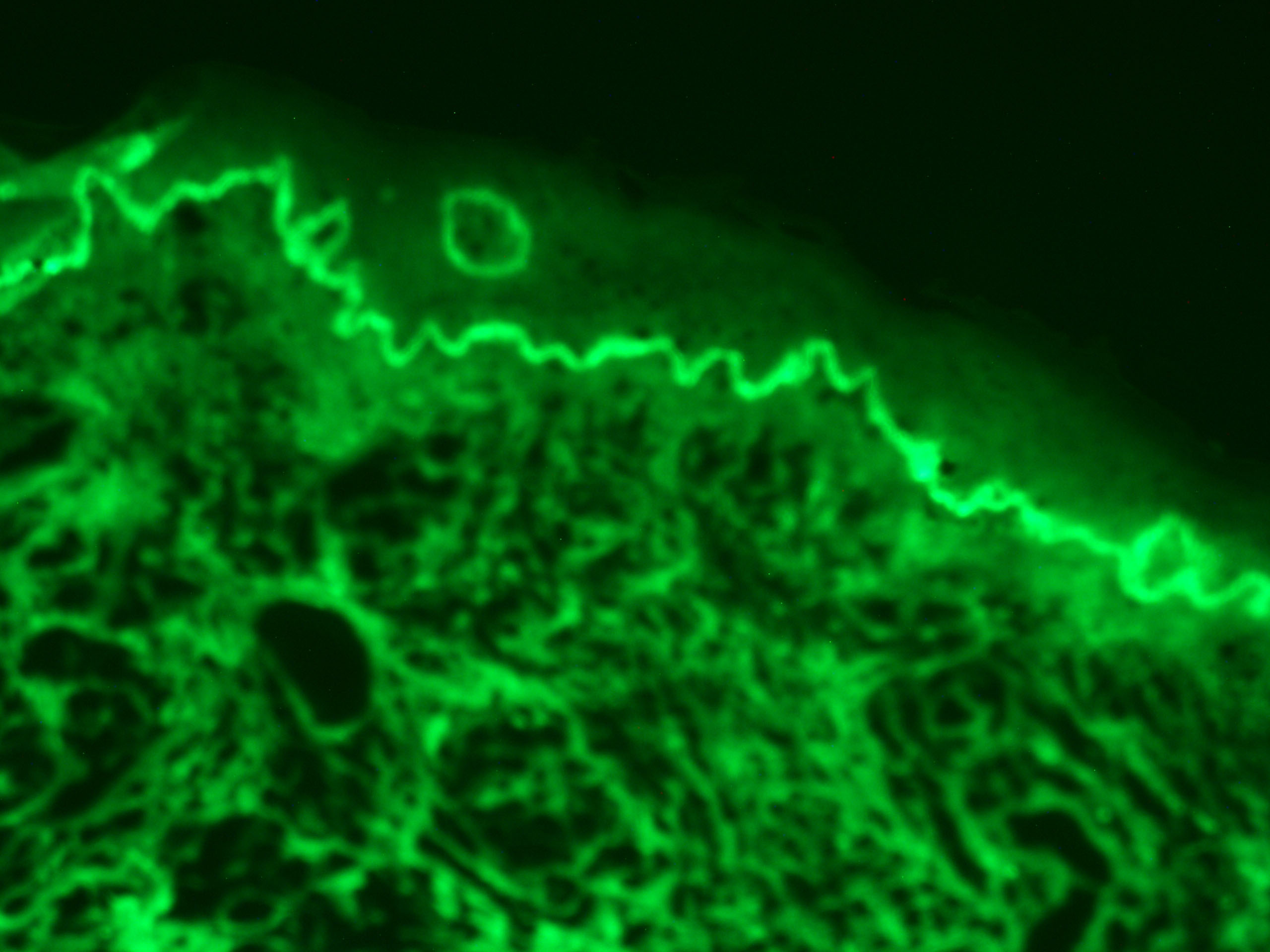
Immunofluorescence can be used on tissue sections, cultured cell lines, or individual cells, and may be used to analyse the distribution of proteins, glycans, and small biological and non-biological molecules. Immunofluoresence can be used in combination with other, non-antibody methods of fluorescent staining, for example, use of DAPI to label DNA. Several microscope designs can be used for analysis of immunofluorescence samples; the simplest is the epifluorescence microscope, and the confocal microscope is also widely used. Various super-resolution microscope designs that are capable of much higher resolution can also be used.
The discipline of Immunoflourescene has developed similarly to Immunohistochemistry. The use of autoantibodies were first detected many years ago on frozen sections of mouse and rat liver. The samples were generally prepared in the laboratory, and the other required reagents, such as conjugates, were rarely available at the optimal concentrations required for the individual diagnostic test systems. Early autoimmune results were thus found to be highly variable. The use of different substrates for autoantibody detection showed that autoantibodies against nuclear components, such as those against double-stranded DNA, anti-dsDNA antibodies, which exhibited a homogeneous immunofluorescence pattern, were easily detectable on liver sections.
Three components of a fluorescence microscope that are different from a light microscope shown below.
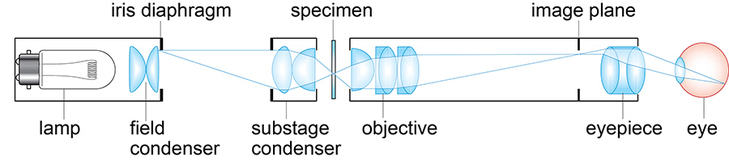
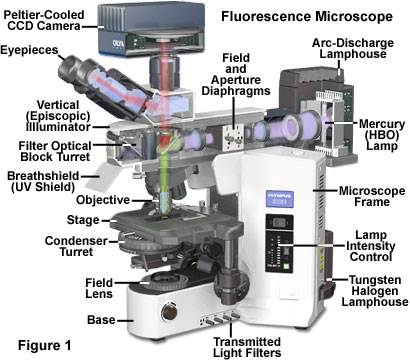
These differences are listed in the table below. These parts of the microscope are shown in the pictures below and how they fit in with the rest of the structures. All the filters are within a filter cube
| Components of Fluorescent Microscope | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Component | Functions | |||||||||||||
| Excitation Filter | The filter is used to make sure that only the light wavelength desired passes through it by blocking other wavelengths from entering the visual field. This is used here to prevent wavelengths, other than the excitation range passing through to the specimen, in the case of fluorescein isothyocynate (FITC) it is 490nm (blue) or tetrametylrhodamine isothiocyanate (TRITC) it is 541nm (Green/Yellow) | |||||||||||||
| Dichroic mirror | This acts as a beam splitter and its function is to separate the light emitted from the unabsorbed excitation light. The excitation light wavelength in FITC is 520nm (Green) or TRITC which is 572nm (Red) | |||||||||||||
| Barrier Filter | This secondary filter allows emitted light to pass, while blocking the unabsorbed light from reaching the eyepiece. | |||||||||||||
These parts of the microscope are shown in the picture below and how they fit in with the rest of the structures. All the filters are within a filter cube
The discipline of Immunoflourescene has developed similarly to Immunohistochemistry. The use of autoantibodies were first detected many years ago on frozen sections of mouse and rat liver. The samples were generally prepared in the laboratory, and the other required reagents, such as conjugates, were rarely available at the optimal concentrations required for the individual diagnostic test systems. Early autoimmune results were thus found to be highly variable. The use of different substrates for autoantibody detection showed that autoantibodies against nuclear components, such as those against double-stranded DNA, anti-dsDNA antibodies, which exhibited a homogeneous immunofluorescence pattern, were easily detectable on liver sections.
Immunofluorescence can be carried out using two different types of staining called direct and Indirect fluorescence. There are advantages and disadvantages to both the techniques shown below.
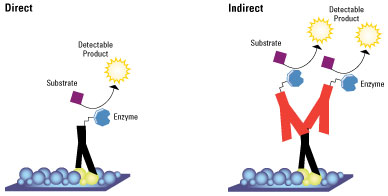
Direct immunoflourscence
Direct Immunofluorescence technique has several advantages over the secondary (or indirect) protocol. One of these is because of the direct conjugation of the antibody to the fluorophore. The lower number of steps in the staining procedure means the process is faster and reduces background signal by avoiding any antibody cross-reactivity or non-specificity. A disadvantage is since the number of fluorescent molecules that can be bound to the primary antibody is limited, direct immunofluorescence is less sensitive than indirect immunofluorescence.
Indirect immunoflourscence
Indirect immunofluorescence however uses two antibodies - the unlabeled first antibody specifically binds the target molecule and a secondary antibody which carries the fluorophore. This recognises the primary antibody and binds to it.
Multiple secondary antibodies can bind a single primary antibody. This provides signal amplification by increasing the number of fluorophore molecules per antigen. This protocol is more complex and time consuming than the direct protocol, but it allows more flexibility because a variety of different secondary antibodies and detection techniques can be used for a given primary antibody.
Immunofluorescence can be carried out using two different types of staining called direct and Indirect fluorescence. There are advantages and disadvantages to both the techniques shown below.

C3c

Direct Immunofluorescence technique has several advantages over the secondary (or indirect) protocol. One of these is because of the direct conjugation of the antibody to the fluorophore. The lower number of steps in the staining procedure means the process is faster and reduces background signal by avoiding any antibody cross-reactivity or non-specificity. A disadvantage is since the number of fluorescent molecules that can be bound to the primary antibody is limited, direct immunofluorescence is less sensitive than indirect immunofluorescence.
Indirect immunoflourscence
Indirect immunofluorescence however uses two antibodies - the unlabeled first antibody specifically binds the target molecule and a secondary antibody which carries the fluorophore. This recognises the primary antibody and binds to it.
Multiple secondary antibodies can bind a single primary antibody. This provides signal amplification by increasing the number of fluorophore molecules per antigen. This protocol is more complex and time consuming than the direct protocol, but it allows more flexibility because a variety of different secondary antibodies and detection techniques can be used for a given primary antibody.